- Here we present the highlights from a recent Biostime Nutrition webinar with guest speaker Dr John Sinn. Dr Sinn is a Paediatric Allergist and Consultant Neonatologist at the Royal North Shore Hospital in Sydney. He has authored extensive publications in allergy prevention, food intolerances, cow’s milk allergy, probiotics and prebiotics, and immunisations. His special interest is in decreasing food allergy sensitivity, via a step ladder approach.
Dr John Sinn received a speaker honorarium from Biostime Nutrition.
This summary was written by Professor Peter SW Davies, Honorary Professor of Childhood Nutrition, University of Queensland. Professor Davies received a writing honorarium from Biostime Nutrition.

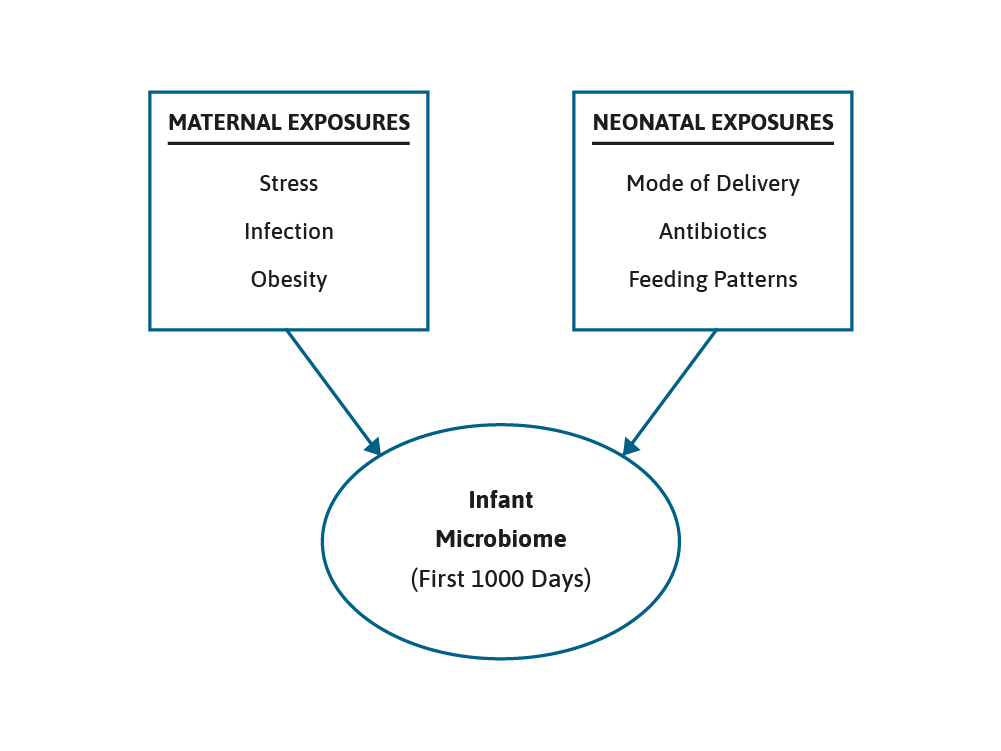
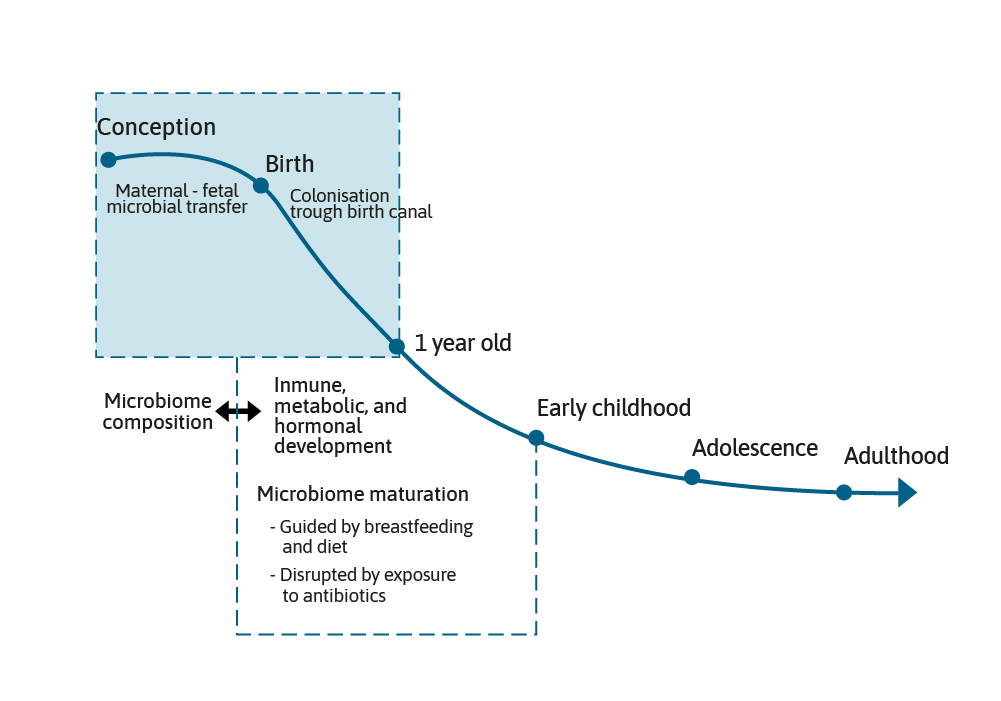
The microbiome grows and develops over the first few years of life and can be impacted by maternal, nutritional, and environmental factors, such as breast feeding or formula feeding, antibiotic use, and the introduction of complementary foods. This is a period of development that is often referred to as the critical “First 1000 Days”.1,2 It is generally accepted that the early gut microbiota found in term, vaginally born and breast fed infants is dominated by two species, Bifidobacterium and Bacteroides.3
The presence of these species has been termed as being characteristic of a healthy infant microbiota. However, before post-natal events shape the microbiome further, an important event for the infant in terms of the initiation of the microbiome and its composition is the mode of delivery.4
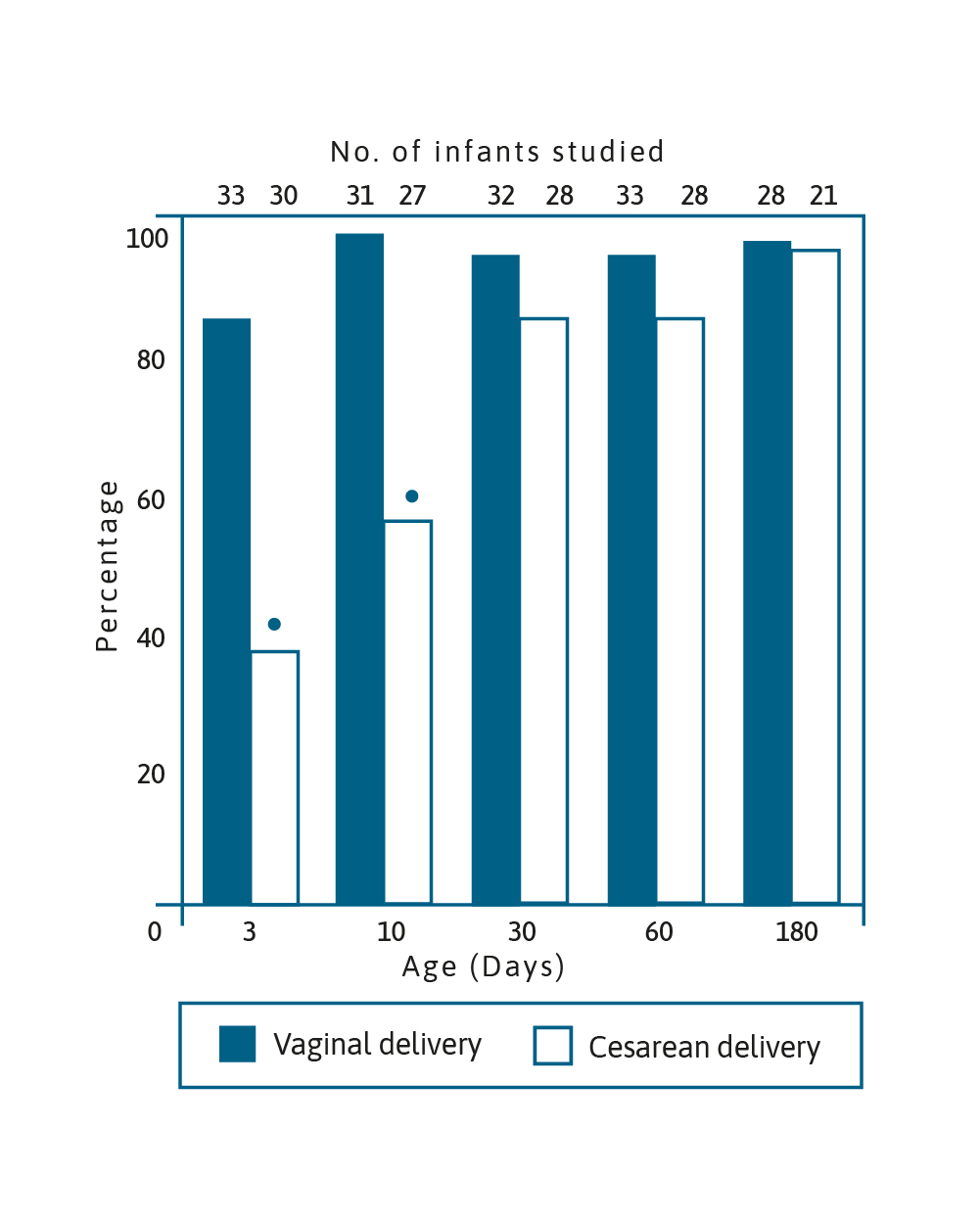
Image adapted from Grolund et al. 1999.5
Percentage of Bifidobacterium-like bacteria colonisation in
infants aged 3, 10, 30, 60 & 180 days born vaginally and by CS
(*p<0.001).5
Over 20 years ago, it was demonstrated that Bifidobacterium colonisation of infants born by caesarean section (CS) was delayed when compared to infants born vaginally. It took around 30 days for the number of CS born infants colonised with Bifidobacterium to reach the percentage of infants born vaginally who were colonised by the bacteria after only 3 days.5 These bacteria are able to metabolise oligosaccharides in breast milk and have an important role in immune function maturation as well as protecting against pathogens in the gut.6 Importantly, birth by CS has been linked to obesity, allergy and diabetes.7–10 Attempts have been made to modify the microbiome in infants born by CS using prebiotics, probiotics and synbiotics. A randomised controlled trial of a synbiotic supplementation showed a higher proportion of Bifidobacterium in the gut from the first week of life until week 8, as well as reduction in Enterobacteriaceace and a lower faecal pH and higher faecal acetate concentration.6 It was concluded that all these changes were emulating the development of the gut environment in vaginally delivered infants.
We know that the use of antibiotics can impact upon the microbiome. For example, administration of ampicillin antenatally, to treat group B Streptococcus, has been shown to significantly reduce the numbers of Bifidobacterium in the infant’s gut at one week of age. Moreover, early antibiotic exposure has been linked to reduced diversity of the infant’s microbiota as well as increased numbers of potentially harmful Proteobacterium, Clostridium and Escherichia Coli bacterium.11 Nevertheless, probiotics have been shown to be helpful in this situation as well. In a recent study, pregnant women were randomly assigned to receive either a probiotic mix, consisting of Bifidobacterium Breve Bb99, Proprionbacterium freundenreichii and Lactobacillus rhamnosus Lc705, and Lactobacillus rhamnosus GG, or a placebo.12 Following the infants birth they also received probiotic supplement or placebo. In the placebo group antibiotic use was strongly related to an altered microbiota, notably a reduction in the abundance of Bifidobacterium. In the infants that received the probiotics, however, the effects of antibiotic use on the microbiota were eliminated or reduced. The use of antibiotics in the first year of life is one of several factors that can interact to increase the risk of functional gastrointestinal disorders, possibly by changing the microbiome.
Such interacting factors include delivery mode, prematurity and feeding pattern at 1 month of age, were investigated in a large, prospective, multicentre study.13 Over a thousand neonates were recruited from 5 neonatal units. The data clearly showed that the use of antibiotics and prematurity were associated with an increased risk of functional gastrointestinal disorders, notably colic and regurgitation. CS and feeding pattern also emerged as additional risk factors for both infant dyschezia and functional.
In recent years, there has been a burgeoning interest in the potential relationship between the microbiome and health and disease.14 Notably, there has been interest in how alterations in the microbiome may be linked to allergy, inflammatory bowel disease (IBD), obesity, diabetes and infantile colic.15 It has been reported that infants with allergies have a relatively higher abundance of Enterobacteriaceae and lower concentrations of Bifidobacterium and Lactobacillus, which are considered beneficial to health.16 These gut bacteria have also been reported to protect the intestinal mucosa from pro-inflammatory cytokines and chemokines in early life, and it has been suggested that individuals with fewer of these advantageous bacteria might have a greater susceptibility to IBD.17 Both obesity and type 2 diabetes have been associated with the microbiome.18,19
Clearly changes to the microbiome may have health benefits and a key role in this area would seem to be prebiotics.
Prebiotics are often defined as non-digestible food ingredients that selectively stimulate the growth of non-pathogenic bacteria in the large intestine. It has long been known that prebiotic consumption may be beneficial to infant health. A recent Cochrane Review showed that prebiotic consumption has a marked effect on the risk of developing eczema, with a highly significant effect on the risk ratio (0.68; 95% CI 0.48–0.97).20 In this example the prebiotic was a mixture of short chain galactooligosaccharides and long-chain fructooligosaccharides, in a ratio of 9:1. These two compounds have long been used as prebiotics in dietetic practice, along with others such as inulin.21 More recently emphasis has moved towards a greater understanding of the role that human milk oligosaccharides (HMOs) may play in the health of infants.
These multifunctional glycans are the third most abundant solid component of human milk after lactose and lipids.22 They consist of five monosaccharides, namely glucose, galactose, N-ethylglucosamine, fucose, and sialic acid. The amounts of HMOs in human milk varies between women and stages of lactation. Colostrum contains around 20–23 g/L HMOs, while the concentration in mature milk is lower at 12–14 g/L.22 The exact structures of the HMOs synthesised by a woman is determined to a large extent by genetic background. An important factor is the Lewis system and secretor status. HMOs are an important prebiotic in humans, digested by beneficial Bifidobacterium in the large intestine, they have a bifidogenic effect.22 HMOs directly influence the immune function of the individual in many ways.
For example, HMOs are known to reduce intestinal crypt cell proliferation, increase barrier function, and inhibit bacterial and viral infections, by binding to pathogens in the gut lumen or inhibiting binding to cell surface glycan receptors.23 There have been a limited numbers of studies, of the potential benefits of adding HMOs to infant formula. The addition of the HMOs, 2’fucosyllactose (2’FL) and lacto-N-neotetraose (LNnt) to full-term infant formula has been approved in many countries worldwide, and trials have confirmed their safety and support of age appropriate growth, as well as being associated with lower morbidity and medication use.24 These HMOs also show great promise in preventing necrotising enterocolitis in pre-term infants.25,26
It has long been known that prebiotic consumption may be beneficial to infant health.
The microbiome appears to have a significant impact on short- and long-term health, including early life events, such CS or antibiotic use, which can disturb the development of the gut bacteria. Ensuring infants and young children develop a “good” microbiome is key to the prevention of allergy and childhood infections. Messaging around the importance of long-term breast feeding should be reinforced, but when breast feeding is not possible, use of infant formula supplemented with prebiotics, probiotics, or synbiotics may be helpful in shaping the microbiome and long-term health outcomes.
- Stiemsma LT & Michels KB. Pediatics 2018;141(4):e20172437 .
- Linner A & Almgren M. Acta Paediatrica 2020;109:443–52 .
- McBurney MI, et al. J Nutr. 2019;149:1882–95 .
- Shao Y, et al. Nature 2019.Doi.org/10.1038/s41586-019-1560-1 .
- Grolund MM, et al. JPGN 1999;28(1):19–25 .
- Chua MC, et al. JPGN 2017;Doi:10.1097/MPG.0000000000001623 .
- Pluyman LPM, et al. J Pediatr 2016;179:111–7e3 .
- Chavarro JE, et al. JAMA Network Open 2020;3(4):e202605 .
- Mitselou N, et al. J Allergy Clin Immunol 2018;142:1510–4 .
- Adeyeye TE, et al. Am J Epidemiol 2019;188(2):355–62 .
- Langdon A, et al. Genome Medicine 2016;8:39 .
- Korpela K, et al. Microbiome 2018;6:182 .
- Salvatore S, et al. J Pediatr 2019;212:44–51 .
- Giles EM & Couper J. J Paeds and Child Health 2020;Doi:10.1111/jpc.14939. 15. Lee YY, et al. J Paeds and Child Health 2017;53:1152–58 .
- Azad M, et al. Clin Exp Allergy 2015;45:632–43 .
- Fava F & Danese S. World J Gastroenterol 2011;17:557–66 .
- Riva A, et al. Environ Microbiol 2017;198(95)95–105 .
- Forslund K, et al. Nature 2015;528(7581):262–6 .
- Osborn DA et al. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No.: CD006474. DOI: 10.1002/14651858.CD006474.pub3 .
- Douglas LC & Sanders ME. J Am Diet Assoc 2008;108:510–21 .
- Wicinski M, et al. Nutrients 2020;12(266): doi:10.3390/nu1210266 .
- Donovan SM & Comstock SS. Ann Nutr Metab 2016;432:62–70 .
- Puccio G, et al. JPGN 2017;64:624–31 .
- Sodhi CP, et al. Ped Res 2020;Doi.org/10.1038/s41390-020-0852-3 .
- Moukarzel S & Bode L. Clin Perinatol 2017;44:193–207 .
- Kukkonen K, et al. J Allergy Clin Immunol 2007;119:192–8 .
- Fox AT, et al. Clin Transl Allergy 2019;9:5 .
- Izumi H, et al. J Nutr 2019;149:344–53 .
-
Stiemsma LT & Michels KB. Pediatics 2018;141(4):e20172437.
-
Linner A & Almgren M. Acta Paediatrica 2020;109:443–52.
-
McBurney MI, et al. J Nutr. 2019;149:1882–95.
-
Shao Y, et al. Nature 2019.Doi.org/10.1038/s41586-019-1560-1.
-
Grolund MM, et al. JPGN 1999;28(1):19–25.
-
Chua MC, et al. JPGN 2017;Doi:10.1097/MPG.0000000000001623.
-
Pluyman LPM, et al. J Pediatr 2016;179:111–7e3.
-
Chavarro JE, et al. JAMA Network Open 2020;3(4):e202605
-
Mitselou N, et al. J Allergy Clin Immunol 2018;142:1510–4.
-
Adeyeye TE, et al. Am J Epidemiol 2019;188(2):355–62.
-
Langdon A, et al. Genome Medicine 2016;8:39.
-
Korpela K, et al. Microbiome 2018;6:182.
-
Salvatore S, et al. J Pediatr 2019;212:44–51..
-
Giles EM & Couper J. J Paeds and Child Health 2020;Doi:10.1111/jpc.14939. 15. Lee YY, et al. J Paeds and Child Health 2017;53:1152–58.
-
Azad M, et al. Clin Exp Allergy 2015;45:632–43.
-
Fava F & Danese S. World J Gastroenterol 2011;17:557–66
-
Riva A, et al. Environ Microbiol 2017;198(95)95–105.
-
Forslund K, et al. Nature 2015;528(7581):262–6.
-
Osborn DA et al. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No.: CD006474. DOI: 10.1002/14651858.CD006474.pub3.
-
Douglas LC & Sanders ME. J Am Diet Assoc 2008;108:510–21.
-
Wicinski M, et al. Nutrients 2020;12(266): doi:10.3390/nu1210266.
-
Donovan SM & Comstock SS. Ann Nutr Metab 2016;432:62–70.
-
Puccio G, et al. JPGN 2017;64:624–31.
-
Sodhi CP, et al. Ped Res 2020;Doi.org/10.1038/s41390-020-0852-3
-
Moukarzel S & Bode L. Clin Perinatol 2017;44:193–207.
-
Kukkonen K, et al. J Allergy Clin Immunol 2007;119:192–8.
-
Fox AT, et al. Clin Transl Allergy 2019;9:5.
-
Izumi H, et al. J Nutr 2019;149:344–53.
Are you a Healthcare Professional?
Important Notice and Declaration
Breast milk is best for babies. Professional advice should be followed before using an infant formula. Introducing partial bottle feeding could negatively affect breast feeding. Good maternal nutrition is important for breast feeding and reversing a decision not to breast feed may be difficult. Infant formula should always be used as directed. Proper use of an infant formula is important to the health of the infant. Social and financial implications should be considered when selecting a method of feeding.
The information provided on this website is intended for use by healthcare professionals only. It is a condition of use of this site that you are a healthcare professional within the meaning of regulations within your country of practice. Then denoting below Yes I am/No icons. For Healthcare Professionals based in Australia : It is a condition of use of this site that you are a healthcare professional within the meaning of the Marketing in Australia of Infant Formulas (MAIF) Agreement or the Therapeutic Goods Act.