- Probiotics comprise many different types of microbes and described by their genus, species, and strain designations. All three components are necessary to identify a probiotic. The full name enables the user to link the specific strain to clinical studies describing its health benefits and safety assessments.
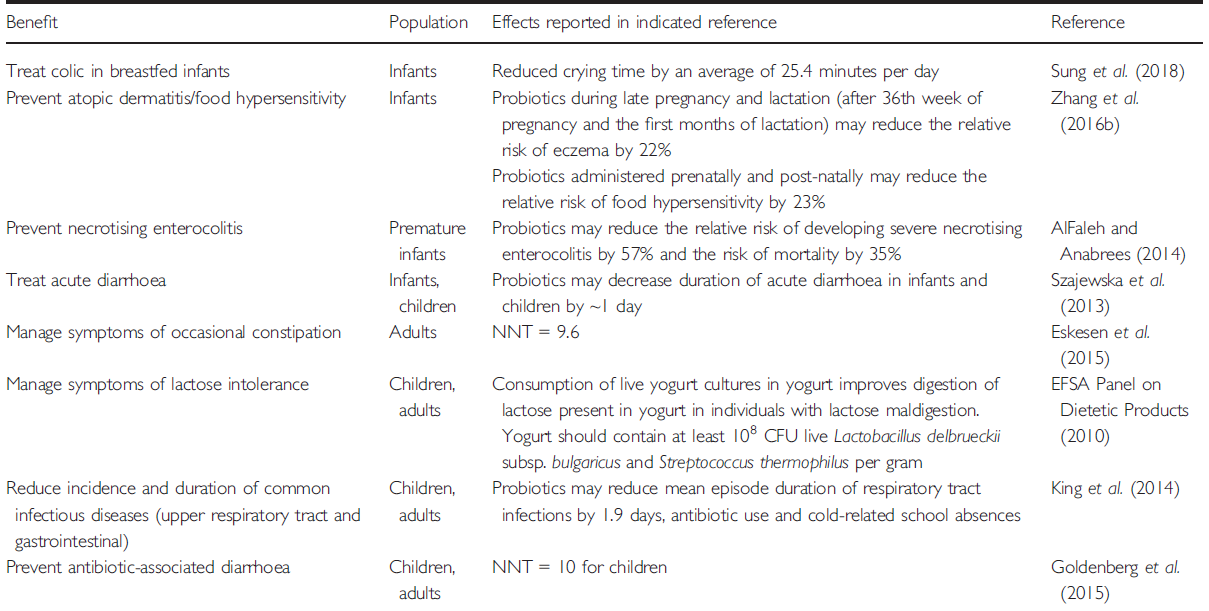
- Current scientific evidence demonstrates benefits of probiotics in infants and children, including for boosting immunity in the prevention or reduction of incidence and duration of common infectious diseases (respiratory tract and gastrointestinal), infantile colic, eczema, constipation, inflammatory bowel diseases, antibiotic-associated diarrhoe and Clostridium difficile infection, necrotising enterocolitis (NEC) in preterm babies.
The International Scientific Association of Probiotics and Prebiotics (ISAPP) has addressed this evolving concept regarding the strain specificity of probiotic effects: that several probiotic mechanisms responsible for certain benefits may be commonly shared amongst most strains of a larger taxonomic group. For example, the production of organic acids, such as lactate and acetate, is shared by most species of both Bifidobacterium and Lactobacillus. These microbial-produced organic acids in the colon provide a range of potential benefits to the intestinal tract and beyond. They play an important role in creating a healthier gut environment by inhibiting undesirable microbes and by cross-feeding other beneficial gut microbes, resulting in production of butyrate, which fuels intestinal epithelial cells. Thus, organic acids produced commonly by many different probiotic strains and species contribute to general gut health benefits.
Despite the existence of shared core mechanisms for probiotic functions; not all probiotic strains are the same. Strain specificity of probiotic benefits is the presumption unless mechanistic and clinical evidence suggests otherwise.
Although there are many characteristics of probiotics microbes, ISAPP’s consensus probiotic definition stipulates: a probiotic must be alive when administered, have a health benefit, and be delivered at an effective dose. A probiotics product also must be safe for the intended use, especially for infants and children who are vulnerable population. It also has to be a defined entity (not an undefined mixture) to allow for appropriate identification to the strain level. The health benefit of probiotics should be substantiated by clinical evidence in adequate amount of colony forming unit (cfu) for the respective target population.
Current scientific evidence demonstrates benefits of probiotics in infants and children, including for boosting immunity in the prevention or reduction of incidence and duration of common infectious diseases (respiratory tract and gastrointestinal), infantile colic, eczema, constipation, inflammatory bowel diseases, antibiotic-associated diarrhoe and Clostridium difficile infection, necrotising enterocolitis (NEC) in preterm babies.
Research suggests that probiotics can be used in an evidence-based manner to address a range of different health concerns. The possible roles of probiotics in reducing disease associated with faulty immune programming leading to autoimmune disorders or correcting dysbiosis that might influence metabolic disorder are also being studies. Research is needed to determine if current or future, so-called next generation probiotics can help address these disorders.
For the healthy infants and children, probiotics may provide a dietary approach to support health and better function of the gut microbiota. As the impact of probiotic interventions on the gut microbiota are further investigated, it may soon be possible to identify likely responders to a specific probiotic, enabling more successful utilization in the future.
At the present time, the high amount and variety of probiotics products can be confusing for patients. The list of frequently asked questions below can be used to help answering the patients’ queries. The overriding need is for patients to be able to recognize products of high quality that address their specific needs. Probiotic quality encompasses safety, potency, and correctness of product labelling, including the accuracy of any health benefit claims, to the extent claims are allowed.
Not necessarily. The recommendation is to use products that have been tested in human studies with positive outcomes for the benefit you are interested in. Sometimes the probiotic product shown to be effective might have a lower dose or fewer strains than another product.
Most studies testing the health benefits of yogurt have been conducted on sweetened yogurts. Therefore, the sugar present in these products does not negate the probiotic effects. However, sweetened yogurts should be consumed only in the context of a healthy, balanced diet.
The stability of the live microbes in a probiotic product depends on conditions of storage. Some products may require refrigeration, but others do not. Responsible product manufacturers make certain that their probiotic is able to meet its label claim through the end of shelf-life if stored as recommended.
Human trials have shown benefits for both food and supplement forms of probiotics, and no comparative trials have been conducted suggesting one format is better than the other. If multiple products of different for¬mats have been shown to be effective, then take the product that best fits with your diet and lifestyle.
Probiotics are live microorganisms beneficial to your health. Prebiotics are not live microbes, but are sub-stances that are used by your beneficial, colonising microorganisms. Simply put, prebiotics are food for your beneficial, native bacteria. Most prebiotics are a type of fibre.
Our bodies are home to trillions of microbes. But remember that we are not uniformly colonised, even throughout the digestive tract. Orally consumed probiotics travel through some sparsely colonised regions of the upper digestive tract, and may be dominant during this passage. Further, even as minor components of the lower digestive tract, a probiotic's metabolic activities can impact the gut environment. Some probiotics have been shown to influence some clinical outcomes, and that's what is important.
Most studies testing the health benefits of yogurt have been conducted on sweetened yogurts. Therefore, the sugar present in these products does not negate the probiotic effects. However, sweetened yogurts should be consumed only in the context of a healthy, balanced diet.
Are you a Healthcare Professional?
Important Notice and Declaration
Breast milk is best for babies. Professional advice should be followed before using an infant formula. Introducing partial bottle feeding could negatively affect breast feeding. Good maternal nutrition is important for breast feeding and reversing a decision not to breast feed may be difficult. Infant formula should always be used as directed. Proper use of an infant formula is important to the health of the infant. Social and financial implications should be considered when selecting a method of feeding.
The information provided on this website is intended for use by healthcare professionals only. It is a condition of use of this site that you are a healthcare professional within the meaning of regulations within your country of practice. Then denoting below Yes I am/No icons. For Healthcare Professionals based in Australia : It is a condition of use of this site that you are a healthcare professional within the meaning of the Marketing in Australia of Infant Formulas (MAIF) Agreement or the Therapeutic Goods Act.